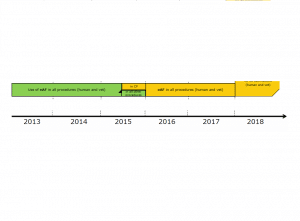
What do I do??
In my circle of friends I feel like I am the “Chandler Bing” and it’s not because I am the funny one; no, it’s because no one knows or understands what I do for a living. My mother still believes I work in a laboratory and have a working knowledge of all medicines and once […]