Update on the future of electronic submissions (eCTD)
Ivowen recently attended the annual EuDRAcon conference in Lörrach, hosted by the German representative, Finkler.
[EuDRAcon is an abbreviation for European Drug Regulatory Affairs Consultants. It is a pan-European network of regulatory affairs consultancy companies dealing with drugs, medical devices, cosmetics and food supplements]
We began the event by voting in the newest member from Iceland, Ymir Vesteinsson (of Day Zero).
Slovenia was chosen to be EuDRAcon president for the next year. The Slovenian representative will host the next conference which is planned for 19th/20th May 2016
We were treated to two very interesting lectures, which sparked off some lively debate.
Dr. Kerstin Stephan (BfArM) discussed with us the topic of “Medicinal Product or Food Supplement? Demarcation from BfArM point of view” and Mr. B.V. Waalwijk introduced “The future of Regulatory data exchange”.
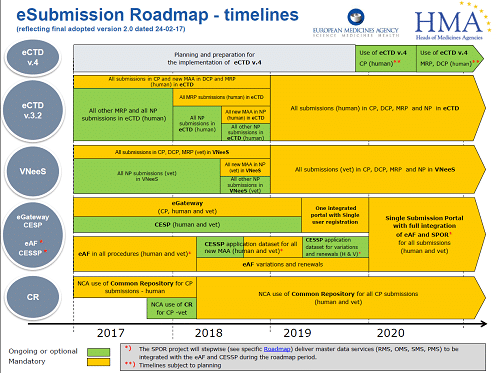
The latter discussion raises important issues for the Pharmaceutical Industry with regard to preparing for the future of electronic submission (as opposed to the digital version of paper files we use today). It is likely in the next 10-15 years that XML databases will begin to replace scanned copies of paper files, bringing us closer to fully compliant electronic dossier.
Ivowen can help you develop, maintain and update your electronic dossier today and into the future. Please contact us for further information.
Written Alice D’Alton