eCTD is coming…
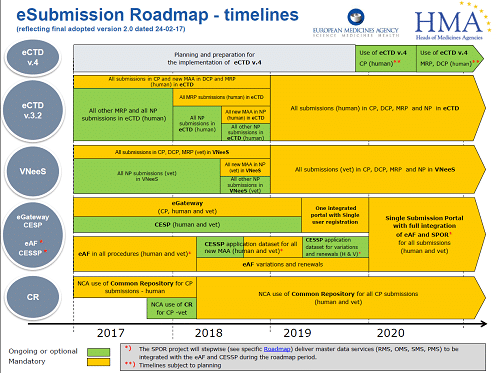
All good (and bad) things, must come to an end. The NeeS (Non-eCTD electronic submissions) submission format, will no longer be accepted for any Marketing Authorisation applications (MAAs) or submissions in MRP or DCP from 1st January 2018. Centralised Procedure (CP) submissions have been mandatory in eCTD since 2010. All new MAAs submitted using either the Decentralised Procedure (DCP) or the Mutual Recognition Procedure (MRP), have had to be in eCTD format since the middle of 2015. As of 1st January 2018, all MRP submissions must be submitted in eCTD. Therefore, if your application/product is not in eCTD format and you need to submit an MRP variation, you have to transition to eCTD for the next submission.
What is the timetable?
Starting from 1st January 2018 it is mandatory for all CP, DCP and MRP submissions to be in eCTD format. For national procedures (NP) the deadline is currently set at 1st July 2018 for new MAAs.
Following quickly behind these requirements, all submissions in CP, DCP, MRP and NP will have to be in eCTD from January 2019. At this stage, the exact date is not confirmed, but you need to be ready.
We can help…
Ivowen can create a baseline dossier for any submission based on currently approved information. Currently, baselines are not mandatory but some Member States are requesting them. In addition, it is best practise to get your dossier into a baseline for all future submissions.
Ivowen can transition your application to eCTD at the next regulatory activity (variation, renewal, Article 61.3, etc.).
Ivowen can manage the eCTD lifecycle for you in-house and provide you with fully valid, submission-ready sequences.
Contact us for more information or for help with building your eCTD now.