New EU MA Applications will require eCTD Submissions by Q3 2015
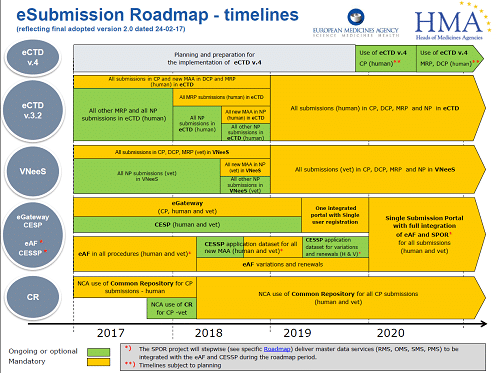
Marketing Authorisation Applications for human use submitted using the Decentralised Procedure (DCP) will need to be provided electronically as eCTD by Q3 2015. Submission in eCTD for Mutual Recognition Procedures (MRP) will also be required by the start of 2017, and finally National Procedures by the start of 2018. eCTD is already required for Centralised Procedures.
The EMA also announced at the end of 2014 that Veterinary Drugs must be filed electronically starting in 2016 for CP/DCP, and all submissions by 2017 by VNeeS (Veterinary Non-eCTD Electronic Submissions).
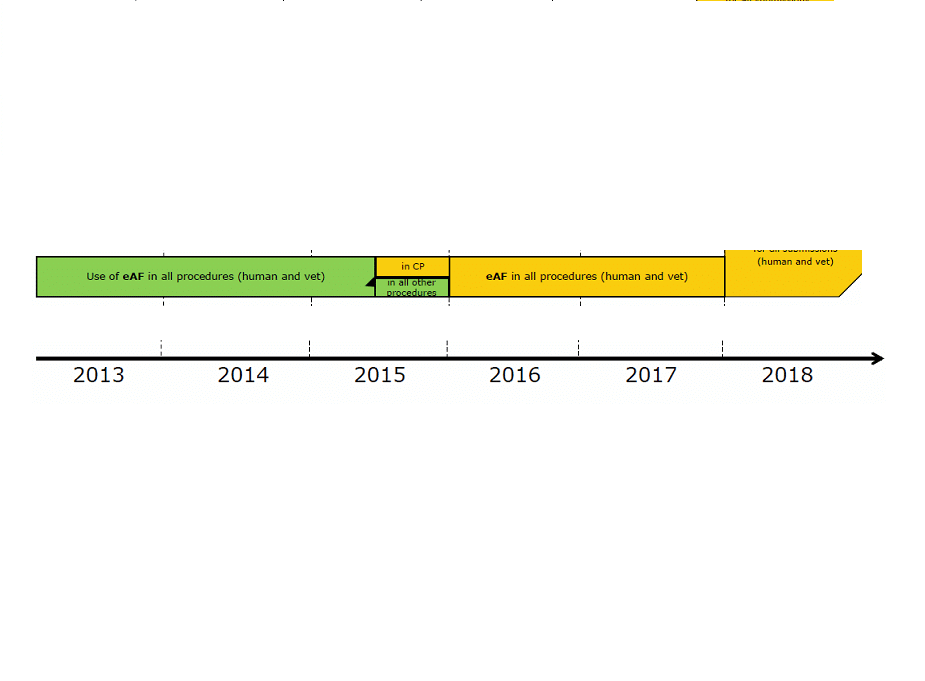
Plans are also underway to replace the Microsoft Word-formatted application form with the eAF (Electronic Application Form) and the deadline will be Q1 2016. Further detailed guidance can be found under the following links:
European Medicines Regulatory Network eSubmission Roadmap
eSubmission Roadmap flow chart
Ivowen are fully eCTD-ready and compliant. Please contact us for more information and for support of your electronic applications.